University of California, San Diego electrical engineers have created experimental solar cells spiked with nanowires that could lead to highly efficient thin-film solar cells of the future.
Indium phosphide (InP) nanowires can serve as electron superhighways that carry electrons kicked loose by photons of light directly to the device’s electron-attracting electrode – and this scenario could boost thin-film solar cell efficiency, according to research recently published in NanoLetters.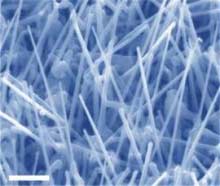
The new design increases the number of electrons that make it from the light-absorbing polymer to an electrode. By reducing electron-hole recombination, the UC San Diego engineers have demonstrated a way to increases the efficiency with which sunlight can be converted to electricity in thin-film photovoltaics.
Including nanowires in the experimental solar cell increased the “forward bias current” – which is a measure of electrical current – by six to seven orders of magnitude as compared to their polymer-only control device, the engineers found.
“If you provide electrons with a defined pathway to the electrode, you can reduce some of the inefficiencies that currently plague thin-film solar cells made from polymer mixtures. More efficient transport of electrons and holes – collectively known as carriers – is critical for creating more efficient solar cells,” said Clint Novotny the first author of the NanoLetters paper, and a recent electrical engineering Ph.D. from UC San Diego’s Jacobs School of Engineering. Novotny is now working on solar technologies at BAE Systems.
Simplified Nanowire Growth
The engineers devised a way to grow nanowires directly on the electrode. This advance allowed them to create the electron superhighways that deliver electrons from the polymer-nanowire interface directly to an electrode.
“If nanowires are going to be used massively in photovoltaic devices, then the growth mechanism of nanowires on arbitrary metallic surfaces is an issue of great importance,” said co-author Paul Yu, a professor of electrical engineering at UC San Diego’s Jacobs School of Engineering. “We contributed one approach to growing nanowires directly on metal.”
The UCSD electrical engineers grew their InP nanowires on the metal electrode – indium tin oxide (ITO) – and then covered the nanowire-electrode platform in the organic polymer, P3HT, also known as poly(3-hexylthiophene). The researchers say they were the first group to publish work demonstrating growth of nanowires directly on metal electrodes without using specially prepared substrates such as gold nanodrops.
“Just a layer of metal can work. In this paper we used ITO, but you can use other metals, including aluminum,” said Paul Yu.
Growing nanowires directly on untreated electrodes is an important step toward the goal of growing nanowires on cheap metal substrates that could serve as foundations for next-generation photovoltaics that conform to the curved surfaces like rooftops, cars or other supporting structures, the engineers say.
“By growing nanowires directly on an untreated electrode surface, you can start thinking about incorporating millions or billions of nanowires in a single device. I think this is where the field is eventually going to end up,” said Novotny. “But I think we are at least a decade away from this becoming a mainstream technology.”
Polymer Solar Cells and Nanowires Meet
As in more traditional organic polymer thin-film solar cells, the polymer material in the experimental system absorbs photons of light. To convert this energy to electricity, each photon-absorbing electron must split apart from its hole companion at the interface of the polymer and the nanowire – a region known as the p-n junction.
Once the electron and hole split, the electron travels down the nanowire – the electron superhighway – and merges seamlessly with the electron-capturing electrode. This rapid shuttling of electrons from the p-n junction to the electrode could serve to make future photovoltaic devices made with polymers more efficient.
“In effect, we used nanowires to extend an electrode into the polymer material,” said co-author Edward Yu, a professor of electrical engineering at UCSD’s Jacobs School of Engineering.
While the electrons travel down the nanowires in one direction, the holes travel along the nanowires in the opposite direction – until the nanowire dead ends. At this point, the holes are forced to travel through a thin polymer layer before reaching their electrode.
Today’s thin-film polymer photovoltaics do not provide freed electrons with a direct path from the p-n junction to the electrode – a situation which increases recombination between holes and electrons and reduces efficiency in converting sunlight to electricity. In many of today’s polymer photovoltaics, interfaces between two different polymers serve as the p-n junction. Some experimental photovoltaic designs do include nanowires or carbon nanotubes, but these wires and tubes are not electrically connected to an electrode. Thus, they do not minimize electron-hole recombination by providing electrons with a direct path from the p-n junction to the electrode the way the new UCSD design does.
Before these kinds of electron superhighways can be incorporated into photovoltaic devices, a series of technical hurdles must be addressed – including the issue of polymer degradation. “The polymers degrade quickly when exposed to air. Researchers around the world are working to improve the properties of organic polymers,” said Paul Yu.
As it was a proof-of-concept project, the UCSD engineers did not measure how efficiently the device converted sunlight to electricity. This explains, in part, why the authors refer to the device in their NanoLetters paper as a “photodiode” rather than a “photovoltaic.”
Having a more efficient method for getting electrons to their electrode means that researchers can make thin-film polymer solar cells that are a little bit thicker, and this could increase the amount of sunlight that the devices absorb.

