Scientists have developed solar cells 200 hundred times thinner than a human hair that they believe will power the nanoscale gadgetry of tomorrow, according to a study released Wednesday.
From consumer devices to bioterrorism monitors to in-body diagnostics, this ultra-microscopic technology is poised to take centre stage in less than a decade from now.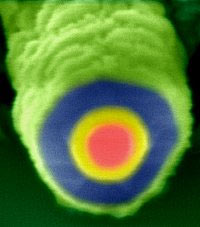
But finding the sources to power it has become a headache.
Charles Leiber and colleagues at Harvard University describe silicon nanowire they devised that can convert light into electrical energy.
Virtually invisible to the naked eye, a single strand can crank out up to 200 picowatts.
Two hundred billionths of a watt may not seem much, but at nanoscale it is enough to provide a steady output of electricity to run ultralow power electronics, including some that could be worn on -- or even inside -- the body.
It is also clean, highly efficient and renewable.
"An individual nanoelectonic device will indeed consume very little power, but to do something interesting will require many interconnected devices and thus the power requirement -- even for nanosystems -- can be a challenge," Lieber explained in an email.
Monitoring bioterrorism threats, for example, would require an entire array of nanosensors, nanoprocessors to analyse the signals received, and nanotransmitters to relay information to a centralised facility, he said.
Conventional sources, he added, are "bulky, non-renewable and expensive" by comparison.
The cable itself looks, at first blush, like the cables used to hook up cable television networks: both have a core covered with two layers, according to the study, published in the British journal Nature.
But the similarity stops there. Beside being 100,000 times smaller, the nanowire is not made of metal but of silicon with three different types of conductivity arranged as layered shells.
Incoming light generates electrons in the outer shell, which are then swept into the second layer and the inner core along micropores.
These "holes", as they are called, carry an equal, but opposite, charge as electrons, which means that the two particles move in opposite directions in the presence of an electric field.
"The electrically connected core and cladding" -- a kind of sheath -- "play the same role as the '+' and '-' termini of a battery," Lieber said.

